Articles
- Page Path
- HOME > STRESS > Volume 25(1); 2017 > Article
-
Review Article
척수 손상에서의 치료적 중재: 약물, 재활, 이식, 심리 치료 - 임우택, 최봉삼
- Current Therapeutic Approaches in Spinal Cord Injury: Pharmacological, Rehabilitation, Cell-based, and Psychological Intervention
- Wootaek Lim, Bongsam Choi
-
Korean Journal of Stress Research 2017;25(1):1-7.
DOI: https://doi.org/10.17547/kjsr.2017.25.1.1
Published online: March 31, 2017
우송대학교, 보건복지대학 물리치료학과
우송대학교, 첨단융합 스포츠재활 연구소
Department of Physical Therapy, College of Health and Welfare, Woosong University, Daejeon, Korea
Advanced Institute of Convergence Sports Rehabilitation, Woosong University, Daejeon, Korea
- Corresponding author Bongsam Choi Department of Physical Therapy, College of Health and Welfare, Woosong University, 171 Dongdaejeon-ro, Dong-gu, Daejeon 34606, Korea Tel: +82-42-630-4622 Fax: +82-42-630-4611 E-mail: bchoi@wsu.ac.kr
• Received: December 29, 2016 • Revised: March 10, 2017 • Accepted: March 10, 2017
Copyright: © The Korean Journal of Stress Research
This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 2,273 Views
- 37 Download
- 3 Crossref
Key messages
- The purpose of this review is to discuss current therapeutic interventions and their limitations for the optimal treatment outcomes of individuals with spinal cord injury (SCI). SCI leads to neurologic deficits and subsequent severe muscle atrophy. In clinics, despite the controversy, pharmacological therapy using methylprednisolone has widely been accepted to reduce additional neurologic deficits caused by a secondary injury such as oxidative stress and lipid peroxidation. Moreover, it facilitates the recovery process. Since the loss of locomotor function may reduce the quality of life for individuals with SCI, many pre-clinical and clinical studies have focused on the recovery of locomotor function. Various forms of locomotor training such as treadmill training, cycle training, and robotic-assisted training are currently available for individuals with SCI. Additionally the cell-based interventions have been receiving much attention as one of potential therapeutic interventions which required further clarifications due to the issues of safety. The physical impairment associated with spinal cord injury may cause an adverse effect on mental health. It is now recommended that combined physical and psychological interventions should be considered to maximize the efficacy of therapeutic interventions.
Abstract
- 이 연구는 현재 척수 손상시 널리 적용되는 치료적 중재에 대한 고찰 및 제한점에 대해 알아보고자 하였다. 척수 손상은 신경학적 손상과 그에 따른 심각한 근육 위축을 초래한다. 손상 초기에는 산화적 스트레스, 지질 과산화 등과 같은 이차적 손상으로 인한 추가 신경 손상을 막기 위해 약물요법이 널리 사용되고 있다. 또한 삶의 질과 관련성이 높은 보행 능력에 대한 임상 전, 임상 연구는 보행 능력의 회복에 그 촛점을 맞추고 있으며, 이에 대한 예로서, 트레드밀 훈련, 자전거 훈련, 로봇 훈련 등이 시행되고 있다. 최근에는 세포나 조직을 이식하는 이식치료가 많은 관심 속에 연구되고 있으나 안전성을 확보하기 위한 추가적인 연구가 필요하다. 척수손상은 단순히 신체손상뿐아니라 정신건강에도 부정적 영향을 주는 다면적인 손상을 초래할 수 있기때문에 단독치료 접근방법 보다는 심리인지적 치료를 포함한 다양한 치료가 결합된 형태를 고려해야 한다.
- In the U.S., it is estimated that 282,000 persons live with spinal cord injury (SCI), with approximately 17,000 new cases each year (Spinal Cord Injury, 2016). The most frequent neurologic categories are incomplete, followed by complete (Spinal Cord Injury, 2016). Since very few people experience complete neurologic recovery, most patients end up requiring extensive physical therapy care, for an extended period of time up to many years. Thus, better understanding of SCI is very important for physical therapist to help, guide and establish rehabilitation strategies.
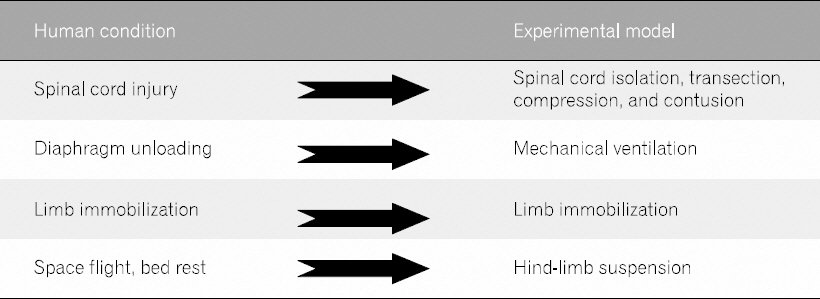
- The analysis of clinical data is complicated by the broad diversity consequences, and the population affected resulting from the injuries. Also, data are not easily collected at the early stage post injury in clinics. For these reasons, experimental models have widely been accepted in preclinical research. The pathophysiological changes in muscles and the preventive strategies for the neurologic deficits following SCI have widely been studied in animal models of SCI (Fig. 1). The safety, feasibility, and effectiveness of therapeutic interventions strongly support the potential for translation of these experimental models for clinical application.
- In traumatic SCI, which is mostly observed in human beings, the overall damage is determined by the combined secondary injury at the molecular and/or cellular levels after primary mechanical insult to the spinal column. The injury caused by external force is inevitable, but it is possible to delay secondary physiological and biological changes using pharmacological interventions. In addition, various physical rehabilitation programs are being applied for rapid physical recovery. Rapid recovery of functional ability can lead to a reduced economic burden of care by shortening hospital length of stay and complications. Finally, recent advances in medical science and technology, along with the development of cell transplantation therapies, are expected to overcome the therapeutic limitations of conventional approaches.
- However, most of the therapeutic approaches currently in use have some limitations, along with the positive effects. This literature review aims to briefly explore and describe current therapeutic interventions and their limitations.
Introduction
- Electronic searches were conducted using EMBASE, MEDLINE, and PubMed to identify relevant literatures published until February 2017. The keywords used to search were (spinal AND cord AND injury), ((pharmacological, rehabilitation, OR cell-based) AND (intervention OR therapy)). The terms were identified in the title or the abstract of journal articles. The studies written in English were included in this review, while Conference abstracts, Case study, and editorial notes were excluded.
Search Strategy
- 1. Pharmacological interventions
- Because of its anti-inflammatory function, steroids have been considered as one of potential interventions in the management of SCI for more than last 40 years. It is presumed that steroids can inhibit or minimize oxidative stress, inflammation, intracellular calcium accumulation, and vascular abnormalities. The efficacy of steroids in the management of SCI was established by National Acute Spinal Cord Injury Studies (NASCIS) I, II, and III. These studies examined methylprednisolone (MP) as a pharmacological treatment intervention in SCI. In NASCIS II and III, significant improvement of sensory and motor function was observed at 6 and 12 months post-injury with MP treatment, which is given within 8 hours of injury (Bracken et al., 1992; Bracken et al., 1990; Bracken et al., 1997; Bracken et al., 1998) (Table 1). However, the findings of these studies are controversial due to issues of statistical analyses and its interpretations. In addition, the potential side effects of methylprednisolone treatment were not taken into consideration while monitoring the effectiveness of steroid therapy in SCI patients (Kwon et al., 2004).
- Summary of National Acute Spinal Cord Injury Study I, II, and III
- In an attempt to halt the detrimental effects of glutamate accumulation following SCI, antagonists of glutamate receptors were used in several studies to inhibit glutamate toxicity and stop the excitotoxicity cascade. Although these drugs provided neuroprotection with subsequent improvement in animal behavior following contusion SCI (Mills et al., 2002), no improvement was seen in human studies. Additionally, calcium- and sodium-channel blockers have been tested as potential treatment interventions in SCI. In animal studies, the calcium channel blockers failed to show any significant improvement (Haghighi et al., 1993). In contrast to calcium blockers, significant improvement in neuroprotective function was observed after treatment with sodium channel blockers in rats (Schwartz et al., 2001; Teng et al., 1997). However, similar benefits of treatment with sodium-channel blockers were not observed in few clinical studies.
- To prevent the programmed cell death following SCI, inhibition of caspases has also been considered as a potential therapeutic intervention. Caspases are proteases involved in apoptosis, necrosis, and inflammation (Eldadah et al., 2000). After administration of minocycline, a significant decrease in caspase-3 and apoptosis was seen in moderate contusion SCI in rats (Festoff et al., 2006). Additionally, in another contusion model of SCI in rats, inhibitiory action of oxidative stress was observed following minocycline administration, specifically lipid peroxidation (Sonmez et al., 2013). Since much more tissue damage is induced by secondary pathophysiological events including oxidative stress and lipid peroxidation, the suppression of oxidative stress plays a key role in the amount of secondary damage.
- Besides the pharmacologic interventions, the use of inhibitors of cyclooxygenase such as Ibuprofen and meclofenamate have been confirmed on improving its function following moderate injury in animal models of SCI (Resnick et al., 1998). Recently, other pharmacologic interventions such as inhibitor of p38, one of mitogen-activated protein kinases, and fluorocarbon have also been reported to be effective for improving locomotor function (Umezawa et al., 2017; Yacoub et al., 2014). In summary, pharmacologic interventions have primarily been focused on minimizing secondary injuries and enhancing locomotor recovery by inhibiting inflammation, free radicals, glutamate accumulation, apoptosis, and demyelination. These factors may hold promise for future clinical trials.
- 2. Rehabilitation interventions
- Significant improvement in muscle properties and function has been reported by implementing activity- based rehabilitation strategies for both incomplete and complete SCI despite the very limited functional recovery in human beings. Strategies of Locomotor training such as treadmill training, cycle training (Fig. 2), and robotic-assisted training take advantage of the central pattern generator to induce movement, and stimulates the spared region of the spinal cord resulting in temporary as well as permanent muscle and spinal cord adaptations. In studies by the use of animal model, even one week of treadmill training after moderately contused SCI have proved that there were increases in muscle fiber CSA and improvements in locomotor function (Stevens et al., 2006). Studies using bicycle training by Bose et al. and Liu et al. had also confirmed similar effects on muscle recovery or locomotor function compared to treadmill training (Bose et al., 2012; Liu et al., 2008). In humans, an increase in muscle CSA was observed in thigh (4.9%) and calf muscle (8.2%) after 12 months of body weight supported treadmill training (BWSTT) (Giangregorio et al., 2006). Similarly, thigh and lower leg muscle CSA were increased to 4.1-56.9% and 3.8-53.6%, respectively after 48 session of BWSTT (Giangregorio et al., 2005). Additionally, improvement of gait speed (180%) and distance (335%) has been reported after BWSTT (12 months, 144 sessions) (Hicks et al., 2005). Hicks et al. also reported that most subjects with SCI maintained walking scores even at 8 months post-training. Thus, locomotor training attenuates muscle atrophy, while it enhances muscle activity and gait quality (Behrman et al., 2000; Giangregorio et al., 2006).
- However, few studies do not support the beneficial effects of physical training after injury. In a study by Fouad et al., 2000, no significant difference in kinematic and locomotor functional tests was observed in rats with incomplete SCI after early treadmill training (Fouad et al., 2000). Similarly, a study by Ung et al., 2010 reported no difference in locomotor function and muscle fiber conversion after treadmill training in complete transection injured mice (Ung et al., 2010). Optimal window of locomotor training with appropriate intensity and duration might influence the effects of training after injury.
- The changes in properties of spinal cord induced by physical activity are relatively less investigated as compared with those caused by other interventions. It has been demonstrated that physical activity in normal adult rat increases brain-derived neurotrophic (BDNF) and nerve growth factor, both which are related to survival of neurons and neural plasticity in various CNS regions (Alderson et al., 1990; Neeper et al., 1996). Further, voluntary exercise increases BDNF and neurotrophic factors in the lumbar spinal cord (Gomez-Pinilla et al., 2001). In rodent SCI, axonal sprouting and regrowth with increased synaptic markers were observed after locomotor training (Goldshmit et al., 2008) and lesion volume was found to be smaller compared to a sedentary group (Andrade et al., 2010). Interestingly, the levels of BDNF mRNA in the brain or lumbar spinal cord significantly correlated with the functional activity in normal adult rats (Gomez-Pinilla et al., 2001). These findings suggest that physical activity after SCI might induce neuro-regeneration and protection.
- Recently, increasing attention has been drawn to the approach that involved electrical stimulation on spinal cord. The communication between brain and the spinal cord below the level of injury is disrupted although motor neurons and nervous tissues are still intact at the levels above and below injury. The application of tonic stimulation at the lumbosacral level of spinal cord has been shown to have positive effects on locomotion and stepping movements, even without supraspinal or sensory input. In addition, tonic epidural stimulation of the dorsum of the spinal cord has been shown to induce stepping movements in decerebrated cats (Gerasimenko et al., 2009) and in complete spinal rats (Ichiyama et al., 2005). The epidural spinal cord stimulation is now known to modulate the excitability of spinal circuits and locomotor function (Alam et al., 2017; Edgerton et al., 2012; Gad et al., 2013).
- 3. Cell-based interventions
- In recent years, cell-based interventions have been introduced and advanced rapidly. This rapid progress will continue to grow (Gu et al., 2017; Lane et al., 2016; Wang et al., 2016; Watzlawick et al., 2016). Transplantation of cells and tissues focuses on the replacement or reconnection of tissue and neuron because conventional approaches showed a limited effect on axonal regeneration and functional recovery. Cell-based intervention began with bone marrow transplantation in the late 1950’s and neural stem cells for neurons were successfully isolated in vitro from the mammalian neural crest and cultured in the early 1990’s (Mathé et al., 1959; Stemple & Anderson, 1992). Since then, various stem cells in vitro were further studied. However, the findings in vitro in the laboratory do not guarantee that the same results can be obtained in vivo because studies in vitro are conducted in a relatively controlled environment and the complex reactions between cells are simplified outside of organisms. With progress in in vitro environment, additional experiments are performed in in vivo and repeated in large animal models of SCI in different species. Cell survival, distribution, migration, and differentiation in vivo can be assessed through various tools. In recent years, the tracking of cells in living organisms has become possible to measure non-invasively through cellular magnetic resonance imaging (Jirjis et al., 2017; Shroff, 2017; Sykova et al., 2007). Once the safety and efficacy have been demonstrated in in vitro and in vivo, clinical trials for application to individuals with SCI are currently available. In many clinical trials, however, ethical issues may hinder the progress and application of the experiments. In addition, unexpected problems can arise with the different physical and physiological characteristics of each patient. In patients with SCI, chronic patients may be considered as preferentially to subacute patients in the phase I clinical trial due to spontaneous recovery at the subacute stage.
- Currently, embryonic, neural, and mesenchymal stem cell are being widely investigated and are likely to expand further in the future. Embryonic stem cells are pluripotent stem cells derived from the inner cell mass of the early embryo (Blair et al., 2011). Those cells are known to be capable of self-renew indefinitely and differentiate all cell types (Puri et al., 2012). The transplant proceeds in vivo after pre-differentiation in vitro (Hendricks et al., 2006). Significant motor recovery and remyelination were commonly reported in a rat model of SCI (Liu et al., 2000; McDonald et al., 1999). In other studies, however, tumor formation was observed after transplantation (Nussbaum et al., 2007; Riess et al., 2007). Neural stem cells are multipotent and used to replace lost tissue after injury. In both in vitro and in vivo, various neurotrophic factors were secreted by neural stem cells (Lu et al., 2003). Improvement of locomotor function, remyelination of axons and normal conduction velocities from the remyelinated axons have been shown (Akiyama et al., 2001; Bottai et al., 2008), but neuronal differentiation limited as transplanted cells remained undifferentiated and is a potential problem. Lastly, mesenchymal stem cells are also multipotent cells. These cells can be easily isolated from bone marrow and adipose. In particular, bone marrow which can be obtained by iliac crest puncture is preferred. Therefore, it is relatively free from ethical issues compared to others. Differentiation in vitro into the neurons, astrocytes, myoblasts, and osteoblasts have been observed (Jiang et al., 2002; Pittenger et al., 1999). Interestingly, neurotropic factors, when combined with transplantation of fetal tissue, were more beneficial as shown by improvement in axonal growth, length of the projection, and number of fiber branches (Bregman et al., 1997). Another beneficial factor of mesenchymal stem cells is an anti-inflammatory effect. The upregulation of transforming growth factor beta 1, an anti- inflammatory factor that controls cell proliferation and apoptosis, has been observed and significantly reduced oxidative stress (Chen et al., 2011; Hawryluk et al., 2012; Kemp et al., 2010; Whone et al., 2012). Unlike others, there are almost no allergic reaction in mesenchymal stem cells. It is known not to cause hypersensitivity reactions (Carrade et al., 2011; Krampera et al., 2003). However, unlike animal studies (Table 2), the efficacy has been reported to be somewhat limited in clinical trials (Syková et al., 2006; Yoon et al., 2007). In conclusion, many studies based on cell transplantation showed significant and valuable changes of neural tissue and motor function, while an understanding of the exact mechanism of recovery or regeneration requires further studies because much still remains unclear and unexpected adverse effects may also occur.
- Mesenchymal stem cell transplantation in an animal model of SCI
- 4. Psychological interventions
- Although many pre-and clinical studies with human and animal models have been widely conducted for the treatment of spinal cord injury, little is known about the psychological intervention. Spinal cord injury does not simply end with physical impairment, but carries an adverse effect on human mental health. Human mental health can directly affect patients’ outcome of treatments. For instance, depression following SCI reduces the effectiveness of treatment and increases the length of hospital stay and treatment (Malec et al., 1983; Tate et al., 1994). In 2011, Fann and colleagues reported 23% of patients with SCI were suffering from depression and only about 29% to 11% of them were on medication or receiving psychotherapy (Fann et al., 2011). Post-traumatic stress disorder (PTSD) is another issue in SCI. Individuals with SCI have been known to experience PTSD at a higher rate. In a study by Radnitz, the current and lifetime prevalence of PTSD were 12% and 29% (Radnitz et al., 1998). Unfortunately, many of them were accompanied by pain (Ullrich et al., 2013). The psychological treatments may have only moderate effects in treating depression (Perkes et al., 2014). Although there is a lack of evidence for the effect on PTSD, psychological treatment may be of some help in relieving pain in patients with the trauma(Mohta et al., 2003). Rather than performing the psychological therapy alone, it may be better to combine cognitive behavioral therapy with psychoeducation (Perkes et al., 2014).
Therapeutic Approaches in Spinal Cord Injury
Table 1
| Study | NASCISa I (1984) | NASCIS II (1990) | NASCIS III (1997) |
|---|---|---|---|
| Number of patients | 330 | 487 | 499 |
| Dosing | MPb 100 mg+25 mg every 6 hours for 10 days or MP 1,000 mg+250 mg every 6 hours for 10 days | MP 30 mg/kg+5.4 mg/kg/hr for 23 hours | MP 5.4 mg/kg for 24 hours or MP 5.4 mg/kg/hr for 48 hours |
| Outcomes | No significant differences between two groups | Significant improvement in motor function | Significant improvement in motor function in individuals treated for 48 hours |
Table 2
| SCI models | Neuronal or axonal regeneration | Sensory improvement | Motor improvement | References |
|---|---|---|---|---|
| Rats, contusion | O | O | Nakajima et al., 2012 | |
| Rats, contusion | O | Karaoz et al., 2012 | ||
| Rats, contusion | O | Cantinieaux et al., 2013 | ||
| Rats, contusion | O | Torres-Espin et al., 2014 | ||
| Rats, contusion | O | Matsushita et al., 2015 | ||
| Rats, compression | O | O | Menezes et al., 2014 | |
| Rats, compression | O | Urdzíková et al., 2014 | ||
| Rats, compression | O | Melo et al., 2016 | ||
| Rats, transection | O | O | Zeng et al., 2015 | |
| Mice, contusion | O | O | Watanabe et al., 2015 | |
| Mice, compression | O | O | Boido et al., 2014 |
- Various therapeutic interventions are effectively and selectively being performed in clinical practice. Each of them has some limitations and it may require attention to application. Incorrect use of hi-dose steroids can be detrimental and aggravate neuronal damage (Hall et al., 2004). It is widely accepted that locomotor training after SCI assists in the qualitative, quantitative, and functional recovery of muscles. However, early locomotor training may not be beneficial, but disadvantageous. The detrimental effects of locomotor training on the spinal cord and muscle may suggest the importance of the optimal time window and appropriate mode of locomotor training by a physical therapist following SCI. Additionally, the cell-based approach is beyond the limits of existing conventional interventions, but unregulated stem cell transplant has the potential risk of tumor formation. It will be important to establish the safety of transplantation strategies before translating the preclinical findings to human beings. In order to maximize the effectiveness of treatment in SCI and ensure safety and feasibility, it is necessary to thoroughly understand each intervention as well as psychological treatment with its limitations.
Conclusion
- Akiyama Y, Honmou O, Kato T, et al. Transplantation of clonal neural precursor cells derived from adult human brain establishes functional peripheral myelin in the rat spinal cord. Exp Neurol. 2001;167(1):27-39.ArticlePubMed
- Alam M, Garcia-Alias G, Jin B, et al. Electrical neuromodulation of the cervical spinal cord facilitates forelimb skilled function recovery in spinal cord injured rats. Exp Neurol. 2017;291:141-150.ArticlePubMedPMC
- Alderson RF, Alterman AL, Barde YA, et al. Brain-derived neurotrophic factor increases survival and differentiated functions of rat septal cholinergic neurons in culture. Neuron. 1990;5(3):297-306.ArticlePubMed
- Andrade MS, Mendonca LM, Chadi G. Treadmill running protects spinal cord contusion from secondary degeneration. Brain Res. 2010;1346:266-278.ArticlePubMed
- Behrman AL, Harkema SJ. Locomotor training after human spinal cord injury: A series of case studies. Phys Ther. 2000;80(7):688-700.ArticlePubMed
- Blair K, Wray J, Smith A. The liberation of embryonic stem cells. PLoS Genet. 2011;7(4):e1002019ArticlePubMedPMC
- Boido M, Garbossa D, Fontanella M, et al. Mesenchymal stem cell transplantation reduces glial cyst and improves functional outcome after spinal cord compression. World Neurosurg. 2014;81(1):183-190.ArticlePubMed
- Bose PK, Hou J, Parmer R, et al. Altered patterns of reflex excitability, balance, and locomotion following spinal cord injury and locomotor training. Front Physiol. 2012;3:258ArticlePubMedPMC
- Bottai D, Madaschi L, Di Giulio AM, et al. Viability-dependent promoting action of adult neural precursors in spinal cord injury. Mol Med. 2008;14(9-10):634-644.ArticlePubMedPMC
- Bracken MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the second national acute spinal cord injury study. N Engl J Med. 1990;322(20):1405-1411.ArticlePubMed
- Bracken MB, Shepard MJ, Collins WF Jr, et al. Methylprednisolone or naloxone treatment after acute spinal cord injury: 1-year follow-up data. Results of the second national acute spinal cord injury study. J Neurosurg. 1992;76(1):23-31.ArticlePubMed
- Bracken MB, Shepard MJ, Holford TR, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the third national acute spinal cord injury randomized controlled trial. National acute spinal cord injury study. Jama 1997;277(20):1597-1604.ArticlePubMed
- Bracken MB, Shepard MJ, Holford TR, et al. Methylprednisolone or tirilazad mesylate administration after acute spinal cord injury: 1-year follow up. Results of the third national acute spinal cord injury randomized controlled trial. J Neurosurg. 1998;89(5):699-706.ArticlePubMed
- Bregman BS, McAtee M, Dai HN, et al. Neurotrophic factors increase axonal growth after spinal cord injury and transplantation in the adult rat. Exp Neurol. 1997;148(2):475-494.ArticlePubMed
- Cantinieaux D, Quertainmont R, Blacher S, et al. Conditioned medium from bone marrow-derived mesenchymal stem cells improves recovery after spinal cord injury in rats: An original strategy to avoid cell transplantation. PLoS One. 2013;8(8):e69515ArticlePubMedPMC
- Cao QL, Zhang YP, Howard RM, et al. Pluripotent stem cells engrafted into the normal or lesioned adult rat spinal cord are restricted to a glial lineage. Exp Neurol. 2001;167(1):48-58.ArticlePubMed
- Carrade DD, Affolter VK, Outerbridge CA, et al. Intradermal injections of equine allogeneic umbilical cord-derived mesenchymal stem cells are well tolerated and do not elicit immediate or delayed hypersensitivity reactions. Cytotherapy. 2011;13(10):1180-1192.ArticlePubMed
- Chen YT, Sun CK, Lin YC, et al. Adipose-derived mesenchymal stem cell protects kidneys against ischemia-reperfusion injury through suppressing oxidative stress and inflammatory reaction. J Transl Med. 2011;9:51ArticlePubMedPMC
- Edgerton VR, Roy RR. A new age for rehabilitation. Eur J Phys Rehabil Med. 2012;48(1):99-109.PubMed
- Eldadah BA, Faden AI. Caspase pathways, neuronal apoptosis, and cns injury. J Neurotrauma. 2000;17(10):811-829.ArticlePubMed
- Fann JR, Bombardier CH, Richards JS, et al. Depression after spinal cord injury: Comorbidities, mental health service use, and adequacy of treatment. Arch Phys Med Rehabil. 2011;92(3):352-360.ArticlePubMed
- Festoff BW, Ameenuddin S, Arnold PM, et al. Minocycline neuroprotects, reduces microgliosis, and inhibits caspase protease expression early after spinal cord injury. J Neurochem. 2006;97(5):1314-1326.ArticlePubMed
- Fouad K, Metz GA, Merkler D, et al. Treadmill training in incomplete spinal cord injured rats. Behav Brain Res. 2000;115(1):107-113.ArticlePubMed
- Gad P, Choe J, Nandra MS, et al. Development of a multi-electrode array for spinal cord epidural stimulation to facilitate stepping and standing after a complete spinal cord injury in adult rats. J Neuroeng Rehabil. 2013;10:2ArticlePubMedPMC
- Gerasimenko Y, Musienko P, Bogacheva I, et al. Propriospinal bypass of the serotonergic system that can facilitate stepping. J Neurosci. 2009;29(17):5681-5689.ArticlePubMedPMC
- Giangregorio L, McCartney N. Bone loss and muscle atrophy in spinal cord injury: Epidemiology, fracture prediction, and rehabilitation strategies. J Spinal Cord Med. 2006;29(5):489-500.ArticlePubMedPMC
- Giangregorio LM, Hicks AL, Webber CE, et al. Body weight supported treadmill training in acute spinal cord injury: Impact on muscle and bone. Spinal Cord. 2005;43(11):649-657.ArticlePubMed
- Giangregorio LM, Webber CE, Phillips SM, et al. Can body weight supported treadmill training increase bone mass and reverse muscle atrophy in individuals with chronic incomplete spinal cord injury? Appl Physiol Nutr Metab. 2006;31(3):283-291.ArticlePubMed
- Goldshmit Y, Lythgo N, Galea MP, et al. Treadmill training after spinal cord hemisection in mice promotes axonal sprouting and synapse formation and improves motor recovery. J Neurotrauma. 2008;25(5):449-465.ArticlePubMed
- Gomez-Pinilla F, Ying Z, Opazo P, et al. Differential regulation by exercise of bdnf and nt-3 in rat spinal cord and skeletal muscle. Eur J Neurosci. 2001;13(6):1078-1084.ArticlePubMed
- Gu C, Li H, Wang C, et al. Bone marrow mesenchymal stem cells decrease chop expression and neuronal apoptosis after spinal cord injury. Neurosci Lett. 2017;636:282-289.ArticlePubMed
- Haghighi SS, Stiens T, Oro JJ, et al. Evaluation of the calcium channel antagonist nimodipine after experimental spinal cord injury. Surg Neurol. 1993;39(5):403-408.ArticlePubMed
- Hawryluk GW, Mothe A, Wang J, et al. An in vivo characterization of trophic factor production following neural precursor cell or bone marrow stromal cell transplantation for spinal cord injury. Stem Cells Dev. 2012;21(12):2222-2238.ArticlePubMed
- Hendricks WA, Pak ES, Owensby JP, et al. Predifferentiated embryonic stem cells prevent chronic pain behaviors and restore sensory function following spinal cord injury in mice. Mol Med. 2006;12(1-3):34-46.ArticlePubMedPMC
- Hicks AL, Adams MM, Martin Ginis K, et al. Long-term body-weight-supported treadmill training and subsequent follow-up in persons with chronic sci: Effects on functional walking ability and measures of subjective well-being. Spinal Cord. 2005;43(5):291-298.ArticlePubMed
- Ichiyama RM, Gerasimenko YP, Zhong H, et al. Hindlimb stepping movements in complete spinal rats induced by epidural spinal cord stimulation. Neurosci Lett. 2005;383(3):339-344.ArticlePubMed
- Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418(6893):41-49.ArticlePubMed
- Jirjis MB, Valdez C, Vedantam A, et al. Diffusion tensor imaging as a biomarker for assessing neuronal stem cell treatments affecting areas distal to the site of spinal cord injury. J Neurosurg Spine. 2017;26(2):243-251.ArticlePubMed
- Karaoz E, Kabatas S, Duruksu G, et al. Reduction of lesion in injured rat spinal cord and partial functional recovery of motility after bone marrow derived mesenchymal stem cell transplantation. Turk Neurosurg. 2012;22(2):207-217.ArticlePubMed
- Kemp K, Hares K, Mallam E, et al. Mesenchymal stem cell-secreted superoxide dismutase promotes cerebellar neuronal survival. J Neurochem. 2010;114(6):1569-1580.ArticlePubMed
- Krampera M, Glennie S, Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific t cells to their cognate peptide. Blood. 2003;101(9):3722-3729.ArticlePubMed
- Kwon BK, Tetzlaff W, Grauer JN, et al. Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J. 2004;4(4):451-464.ArticlePubMed
- Lane MA, Lepore AC, Fischer I. Improving the therapeutic efficacy of neural progenitor cell transplantation following spinal cord injury. Expert Rev Neurother. 2017;17(5):433-440.ArticlePubMed
- Liu M, Bose P, Walter GA, et al. A longitudinal study of skeletal muscle following spinal cord injury and locomotor training. Spinal Cord. 2008;46(7):488-493.ArticlePubMed
- Liu S, Qu Y, Stewart TJ, et al. Embryonic stem cells differentiate into oligodendrocytes and myelinate in culture and after spinal cord transplantation. Proc Natl Acad Sci U S A. 2000;97(11):6126-6131.ArticlePubMedPMC
- Lu P, Jones LL, Snyder EY, et al. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol. 2003;181(2):115-129.ArticlePubMed
- Malec J, Neimeyer R. Psychologic prediction of duration of inpatient spinal cord injury rehabilitation and performance of self-care. Arch Phys Med Rehabil. 1983;64(8):359-363.PubMed
- Mathe G, Jammet H, Pendic B, et al. [transfusions and grafts of homologous bone marrow in humans after accidental high dosage irradiation]. Rev Fr Etud Clin Biol. 1959;4(3):226-238.PubMed
- Matsushita T, Lankford KL, Arroyo EJ, et al. Diffuse and persistent blood-spinal cord barrier disruption after contusive spinal cord injury rapidly recovers following intravenous infusion of bone marrow mesenchymal stem cells. Exp Neurol. 2015;267:152-164.ArticlePubMed
- McDonald JW, Liu XZ, Qu Y, et al. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat Med. 1999;5(12):1410-1412.ArticlePubMed
- Melo FR, Bressan RB, Forner S, et al. Transplantation of human skin-derived mesenchymal stromal cells improves locomotor recovery after spinal cord injury in rats. Cell Mol Neurobiol. 2016.Article
- Menezes K, Nascimento MA, Goncalves JP, et al. Human mesenchymal cells from adipose tissue deposit laminin and promote regeneration of injured spinal cord in rats. PLoS One. 2014;9(5):e96020ArticlePubMedPMC
- Mills CD, Hulsebosch CE. Increased expression of metabotropic glutamate receptor subtype 1 on spinothalamic tract neurons following spinal cord injury in the rat. Neurosci Lett. 2002;319(2):59-62.ArticlePubMed
- Mohta M, Sethi AK, Tyagi A, et al. Psychological care in trauma patients. Injury. 2003;34(1):17-25.ArticlePubMed
- Nakajima H, Uchida K, Guerrero AR, et al. Transplantation of mesenchymal stem cells promotes an alternative pathway of macrophage activation and functional recovery after spinal cord injury. J Neurotrauma. 2012;29(8):1614-1625.ArticlePubMedPMC
- Neeper SA, Gomez-Pinilla F, Choi J, et al. Physical activity increases mrna for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726(1-2):49-56.ArticlePubMed
- Nussbaum J, Minami E, Laflamme MA, et al. Transplantation of undifferentiated murine embryonic stem cells in the heart: Teratoma formation and immune response. Faseb. 2007;21(7):1345-1357.Article
- Perkes SJ, Bowman J, Penkala S. Psychological therapies for the management of co-morbid depression following a spinal cord injury: A systematic review. J Health Psychol. 2014;19(12):1597-1612.ArticlePubMed
- Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143-147.ArticlePubMed
- Puri MC, Nagy A. Concise review: Embryonic stem cells versus induced pluripotent stem cells: The game is on. Stem Cells. 2012;30(1):10-14.ArticlePubMed
- Radnitz CL, Hsu L, Willard J, et al. Posttraumatic stress disorder in veterans with spinal cord injury: Trauma-related risk factors. J Trauma Stress. 1998;11(3):505-520.ArticlePubMed
- Resnick DK, Graham SH, Dixon CE, et al. Role of cyclooxygenase 2 in acute spinal cord injury. J Neurotrauma. 1998;15(12):1005-1013.ArticlePubMed
- Riess P, Molcanyi M, Bentz K, et al. Embryonic stem cell transplantation after experimental traumatic brain injury dramatically improves neurological outcome, but may cause tumors. J Neurotrauma. 2007;24(1):216-225.ArticlePubMed
- Schwartz G, Fehlings MG. Evaluation of the neuroprotective effects of sodium channel blockers after spinal cord injury: Improved behavioral and neuroanatomical recovery with riluzole. J Neurosurg. 2001;94(2 Suppl):245-256.ArticlePubMed
- Shihabuddin LS, Horner PJ, Ray J, et al. Adult spinal cord stem cells generate neurons after transplantation in the adult dentate gyrus. J Neurosci. 2000;20(23):8727-8735.ArticlePubMedPMC
- Shroff G. Magnetic resonance imaging tractography as a diagnostic tool in patients with spinal cord injury treated with human embryonic stem cells. Neuroradiol J. 2017;30(1):71-79.ArticlePubMedPMC
- Sonmez E, Kabatas S, Ozen O, et al. Minocycline treatment inhibits lipid peroxidation, preserves spinal cord ultrastructure, and improves functional outcome after traumatic spinal cord injury in the rat. Spine (Phila Pa 1976). 2013;38(15):1253-1259.ArticlePubMed
- Spinal cord injury (SCI) 2016 facts and figures at a glance. J Spinal Cord Med. 2016;39(4):493-494.PubMedPMC
- Stemple DL, Anderson DJ. Isolation of a stem cell for neurons and glia from the mammalian neural crest. Cell. 1992;71(6):973-985.ArticlePubMed
- Stevens JE, Liu M, Bose P, et al. Changes in soleus muscle function and fiber morphology with one week of locomotor training in spinal cord contusion injured rats. J Neurotrauma. 2006;23(11):1671-1681.ArticlePubMed
- Sykova E, Homola A, Mazanec R, et al. Autologous bone marrow transplantation in patients with subacute and chronic spinal cord injury. Cell Transplant. 2006;15(8-9):675-687.ArticlePubMed
- Sykova E, Jendelova P. In vivo tracking of stem cells in brain and spinal cord injury. Prog Brain Res. 2007;161:367-383.ArticlePubMed
- Tate D, Forchheimer M, Maynard F, et al. Predicting depression and psychological distress in persons with spinal cord injury based on indicators of handicap. Am J Phys Med Rehabil. 1994;73(3):175-183.ArticlePubMed
- Teng YD, Wrathall JR. Local blockade of sodium channels by tetrodotoxin ameliorates tissue loss and long-term functional deficits resulting from experimental spinal cord injury. J Neurosci. 1997;17(11):4359-4366.ArticlePubMedPMC
- Torres-Espin A, Redondo-Castro E, Hernandez J, et al. Bone marrow mesenchymal stromal cells and olfactory ensheathing cells transplantation after spinal cord injury--a morphological and functional comparison in rats. Eur J Neurosci. 2014;39(10):1704-1717.ArticlePubMed
- Ullrich PM, Smith BM, Poggensee L, et al. Pain and post-traumatic stress disorder symptoms during inpatient rehabilitation among operation enduring freedom/operation iraqi freedom veterans with spinal cord injury. Arch. Phys Med Rehabil. 2013;94(1):80-85.Article
- Umezawa H, Naito Y, Tanaka K, et al. Genetic and pharmacological inhibition of p38alpha improves locomotor recovery after spinal cord injury. Front Pharmacol. 2017;8:72PubMedPMC
- Ung RV, Lapointe NP, Rouleau P, et al. Non-assisted treadmill training does not improve motor recovery and body composition in spinal cord-transected mice. Spinal Cord. 2010;48(10):750-755.ArticlePubMed
- Urdzikova LM, Ruzicka J, LaBagnara M, et al. Human mesenchymal stem cells modulate inflammatory cytokines after spinal cord injury in rat. Int J Mol Sci. 2014;15(7):11275-11293.ArticlePubMedPMC
- Wang TG, Xu J, Zhu AH, et al. Human amniotic epithelial cells combined with silk fibroin scaffold in the repair of spinal cord injury. Neural Regen Res. 2016;11(10):1670-1677.ArticlePubMedPMC
- Watanabe S, Uchida K, Nakajima H, et al. Early transplantation of mesenchymal stem cells after spinal cord injury relieves pain hypersensitivity through suppression of pain-related signaling cascades and reduced inflammatory cell recruitment. Stem Cells. 2015;33(6):1902-1914.ArticlePubMed
- Watzlawick R, Rind J, Sena ES, et al. Olfactory ensheathing cell transplantation in experimental spinal cord injury: Effect size and reporting bias of 62 experimental treatments: A systematic review and meta-analysis. PLoS Biol. 2016;14(5):e1002468ArticlePubMedPMC
- Whone AL, Kemp K, Sun M, et al. Human bone marrow mesenchymal stem cells protect catecholaminergic and serotonergic neuronal perikarya and transporter function from oxidative stress by the secretion of glial-derived neurotrophic factor. Brain Res. 2012;1431:86-96.ArticlePubMed
- Yacoub A, Hajec MC, Stanger R, et al. Neuroprotective effects of perflurocarbon (oxycyte) after contusive spinal cord injury. J Neurotrauma. 2014;31(3):256-267.ArticlePubMedPMC
- Yoon SH, Shim YS, Park YH, et al. Complete spinal cord injury treatment using autologous bone marrow cell transplantation and bone marrow stimulation with granulocyte macrophage-colony stimulating factor: Phase i/ii clinical trial. Stem Cells. 2007;25(8):2066-2073.ArticlePubMed
- Zeng X, Qiu XC, Ma YH, et al. Integration of donor mesenchymal stem cell-derived neuron-like cells into host neural network after rat spinal cord transection. Biomaterials. 2015;53:184-201.ArticlePubMed
References
Figure & Data
References
Citations
Citations to this article as recorded by 

- Developing a Psychological Intervention for Decreasing Bedtime Procrastination: The BED-PRO Study
Sooyeon Suh, Nayoung Cho, Sonhye Jeoung, Hyeyoung An
Behavioral Sleep Medicine.2022; 20(6): 659. CrossRef - A Case Report of Complex Korean Medicine Treatments for Tetraplegia Caused by Spinal Cord Injury
Eun-jung Kim, Dong-hoon Kim, Sang-gu Yoo, Da-hye Kim, Se-won Lee, Ji-yun Bae, Seon-woo Kim, Cheol-woo Park, Shin-chul Hur
The Journal of Internal Korean Medicine.2020; 41(2): 122. CrossRef - Does vitamin C have the ability to augment the therapeutic effect of bone marrow-derived mesenchymal stem cells on spinal cord injury?
Nesrine Salem, MohamedY Salem, MohammedM Elmaghrabi, MoatazA Elawady, MonaA Elawady, Dina Sabry, Ashraf Shamaa, Abdel-HaleemH Elkasapy, Noha Ibrhim, Azza Elamir
Neural Regeneration Research.2017; 12(12): 2050. CrossRef



 PubReader
PubReader Cite
Cite